On January 31, the TPE24 (Total Product Expo) tobacco show will open in Las Vegas, Nevada. According to the two supreme statistics, the number of enterprises related to atomized electronic cigarettes in this exhibition exceeds 200. According to the exhibitor list, the two supremacy also noted that the FDA Center for Tobacco products, the direct regulatory department of electronic cigarettes in the United States, will also participate in the exhibition, booth number 10176, located in the e-cigarette booth concentration area.
According to previous experience, regulatory authorities in Italy, Germany, Indonesia and other countries have participated in e-cigarette shows. These regulators usually participate in one of two ways. One is similar to the way the FDA participated in TPE24, setting up a booth to observe market dynamics and conduct regulatory advocacy activities. The other way is not to set up a booth, but they will enter the exhibition for inspection, in this case, there is usually on-site law enforcement to investigate and punish illegal electronic cigarettes and related products.
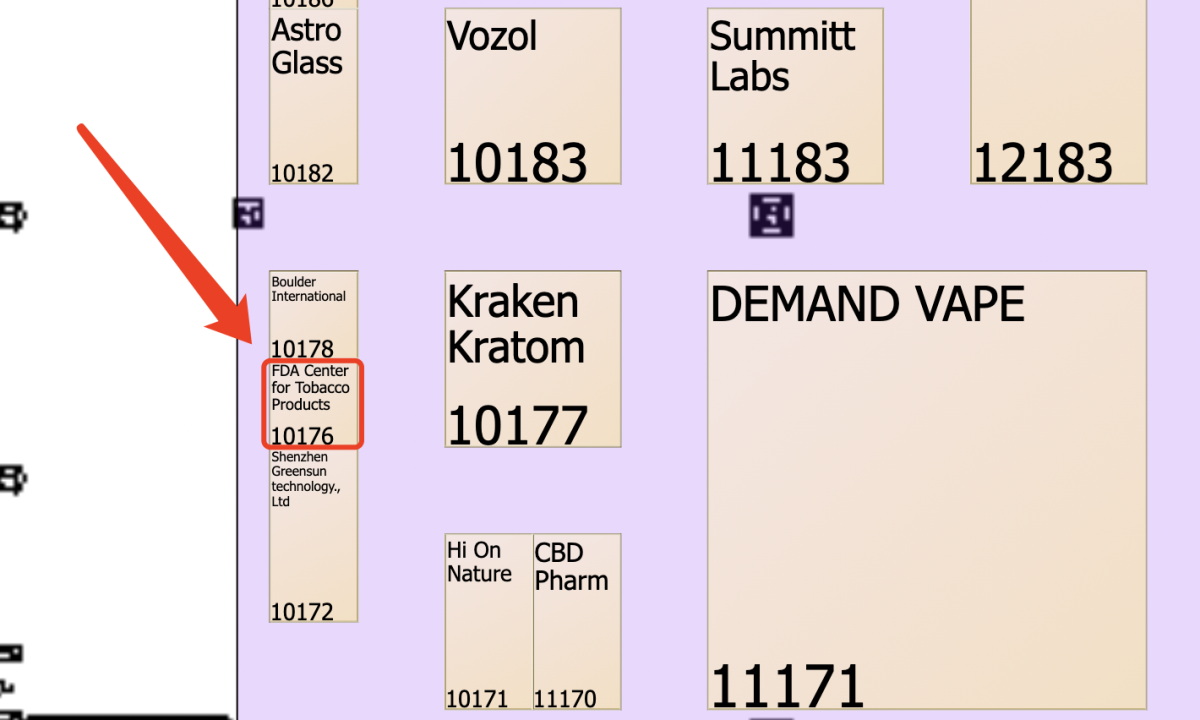
It is not possible to determine the specific purpose of FDA’s participation in the exhibition, but the two generals will pay close attention at the exhibition site and report relevant information for readers in a timely manner.
In addition, after the exhibition, 2FIRSTS will hold the TPE exhibition and in-depth sharing of the US market on February 6. At that time, Hou Yuhan, global executive editor of 2FIRSTS, and the US team will share the latest exhibition findings and in-depth discussion of US product trends and policy trends. For more information and to register, please contact 2FIRSTS staff by scanning the QR code below.

.jpg)







